Why Type of Chemistry Matters, and What Is Available?
The type of chemistry a user chooses for their BESS system can affect a variety of elements from energy density to safety. There are many options available; this section describes lithium-ion, lead acid, sodium sulfur, flow batteries, and hydrogen.
Understanding the required energy can help you select appropriate chemistries or designs. It is also important to consider the cost and safety of the system being chosen.
Main Points
- When deciding what BESS system to use, it is important to consider the required energy demand, required energy duration, cost, and safety in addition to specific regional factors (e.g., humidity, temperature, and temperature variability).
- Lithium-ion, lead acid, and vanadium redox flow batteries are most commonly available for various sizes and durations.
- Vanadium redox flow batteries tend to be more expensive than alternative options at short durations and are not very energy dense, so even at more cost-efficient sizes they can occupy significant land space.
- Lithium-ion solutions offer a more energy-dense alternative; however, there is increased safety risk because of thermal runaway (a chain reaction where an increase in temperature leads to further temperature rise, potentially causing the battery to overheat and fail). Lead acid batteries offer a less energy-dense alternative.
First, Consider This
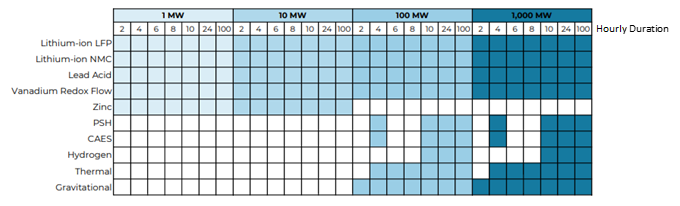
Pacific Northwest National Laboratory (PNNL) conducted a Grid Energy Storage Technology Cost and Performance Assessment in 2022. One of the key figures from this publication, shown as Figure 1, depicts power capacity and energy duration. This is important because it allows someone to identify which technology might be available for their need. Based on the figure, lithium-ion, lead acid, and vanadium redox flow batteries are most widely available. For example, if you need 95 megawatts (MW) of power and must operate for seven hours, the most applicable chemistries/technologies available to you are lithium iron phosphate (LFP), nickel manganese cobalt (NMC), and lead acid. Another example with a smaller system would be if you need 500 kilowatts (kW) of power to operate at most for one hour — in this case, LFP, NMC, lead acid, vanadium redox flow, and zinc are available.
Next, Consider This
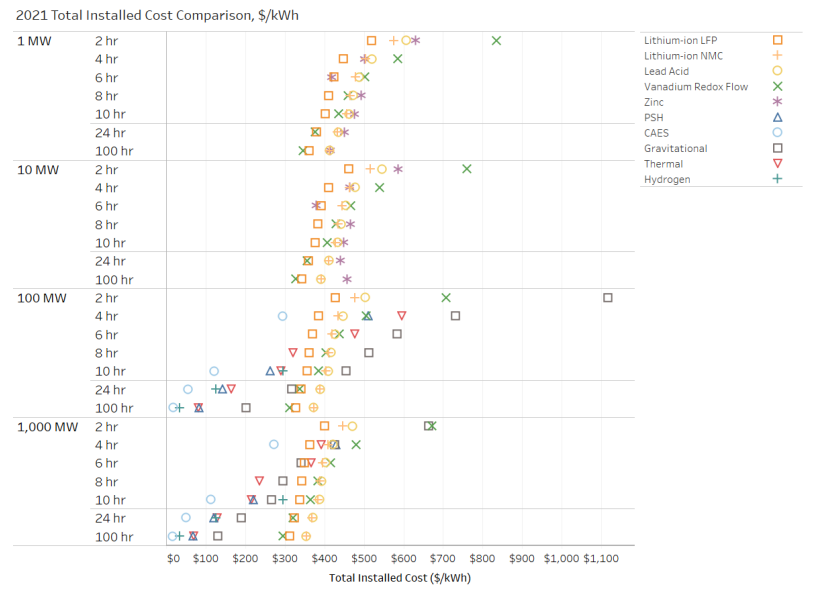
In the same study, PNNL presented the following figure (Figure 2) on costing analysis. As expected, as the system grows, it gets cheaper per kilowatt hour (kWh). One of the noticeable trends here is that vanadium flow batteries tend to be more expensive than other technologies for all sizes and durations. Lithium-ion technology can be more expensive than other alternatives for long durations.
Now, Read This
A National Renewable Energy Laboratory (NREL) study, Global Overview of Energy Storage Performance Test Protocols, briefly compared technologies as well. When distinguishing between lithium-ion and lead-acid batteries, the study had this to say:
“Lead-acid and lithium-ion were the most common batteries utilized, and between those two, lithium-ion energy storage systems tend to perform better than lead-acid at extreme conditions (particularly hot) and offer a longer life. Lead-acid batteries are less prone to thermal runaway, making them slightly safer.”
Text excerpt from page 6 of https://www.nrel.gov/docs/fy21osti/77621.pdf
Finally, Read This
Regarding vanadium flow and sodium sulfur batteries, a Stanford publication had this to say:
“Sodium sulfur batteries have a fairly low cost…making them an economically viable solution. Unfortunately, the fact that they run at such a high temperature makes them slightly unsafe, and they use toxic materials which mean that once their use is up they are difficult to discard…
flow batteries have a low cost comparable to sodium sulfur batteries at 500 kwh, and they are easy to scale. This is because if you want to double the size of a traditional battery, you have to double the price (because you essentially are just getting two units), but with a flow battery you simply increase the tank size which means that the price per kwh goes down much more than the price increases. Unfortunately flow batteries are not very energy dense, which means they require more land to employ.”
Text excerpt from http://large.stanford.edu/courses/2016/ph240/smith-c1/

